What is Human Factors and Usability Validation?
FDA uses human factor and usability validation guidance document to assist studies how people interact with systems. Medical device manufacturer, pharmaceutical concern producing a drug delivery system such as an injector or inhaler, follow appropriate human factors and usability engineering processes. Human factor studies play an integral role in your concern to maximize the likelihood that new medical devices will be safe and effective for the intended users, uses and use environments.
Human factors or usability engineering processes are to be followed during the development of new medical devices, focusing specifically on the user interface, where the user interface includes all points of interaction between the product and the user(s) including elements such as displays, controls, packaging, product labels, instructions for use, etc. FDA continues to focus on the importance of conducting human factors studies for medical devices prior to premarket submissions.Its Importance
The influx of recent FDA discussions and robust guidance documents are on the heels of several reports of life-threatening device user errors. Human Factors studies provide validity and value to medical device regulatory submissions. Human factors / usability validation is very different from device validation, Click here to learn more in this session.
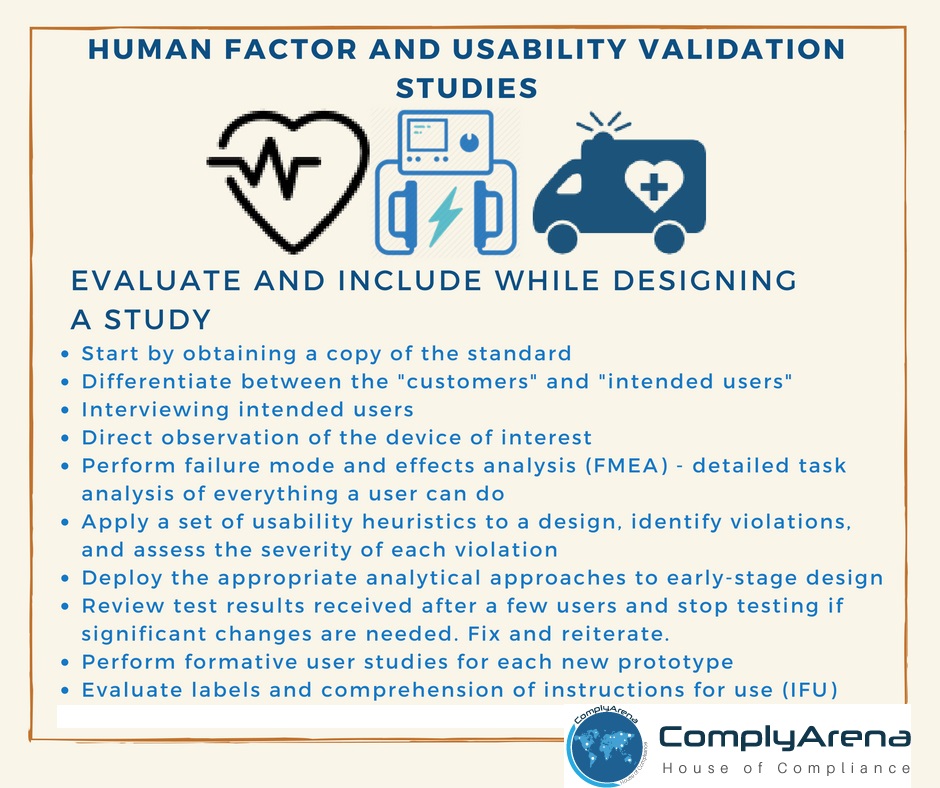
These human factor studies encourage manufacturers to:
- Improve the safety of medical devices and equipment to reduce user error
- Improve the design of medical devices to minimize potential use errors
- Help careful design of the user interface, the hardware and software features that define the interaction between users and equipment
- Better evaluate safety, effectiveness, and substantial equivalence of medical devices
- To save time, money, and resources in the long-run by application of these validation procedures from the start
- Understand the feedback from human factor tests and design easier-to-use devices
- Design and develop safer connections between device components and accessories (e.g., power cords, leads, tubing, cartridges)
- Help ensure reduce user reliance on user manuals, and user training and retraining
Application of Human Factors and Usability Validation Tests
FDA has recommended that medical device manufacturers and clinical researchers design human factor and usability validation studies, should almost “test drive” their products in real-life circumstances, requiring closer interaction with patients and consideration of their feedback.Human factor and usability validation studies may seem complicated, but it could be summarized as below:
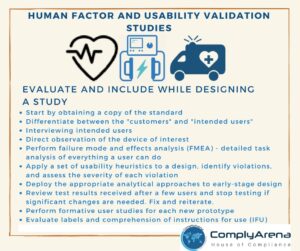
Ensure you evaluate and include the below while designing a study
- Start by obtaining a copy of the standard. You can buy the standard from ISO
- Differentiate between the “Customers” and “Intended Users”, Click here to learn more in this session.
- Interviewing intended users
- Direct observation of the device of interest
- Perform failure mode and effects analysis (FMEA) – detailed task analysis of everything a user can do
- Apply a set of usability heuristics to a design, identify violations, and assess the severity of each violation
- Deploy the appropriate analytical approaches to early-stage design
- Review test results received after a few users and stop testing if significant changes are needed. Fix and reiterate
- Perform formative user studies for each new prototype
- Evaluate labels and comprehension of instructions for use (IFU)
You may need to extend your Usability Engineering Process to include non-electrical devices and interfaces
Ensure your in-house usability team is familiar with the changing FDA requirements
Summary :
In the USA, the Food and Drug Administration (FDA) has recognized ISO/IEC 62366 and has issued a list describing the minimum documentation that must accompany any declaration of conformance to the standard. Incorporating human factors early and often throughout the design can result in a better, safer, and more usable design. Early incorporation ensures that you can avoid making changes to designs late in the development process and, in worst cases, the high costs associated with device recalls.
Ensure your in-house usability team is familiar with the requirements in the standard and draw up a plan for Human factor and usability validation Process in place. You can also find an experienced usability consultant who can help you understand the details of the standard and can work with you to implement the requirements. Always keep your in-house team updated with the latest from FDA.